Aurobindo’s generic Adalat CC gets FDA OK
The market size of the product was $95 million for the 12-month period ended May 2021, according to IQVIA data.
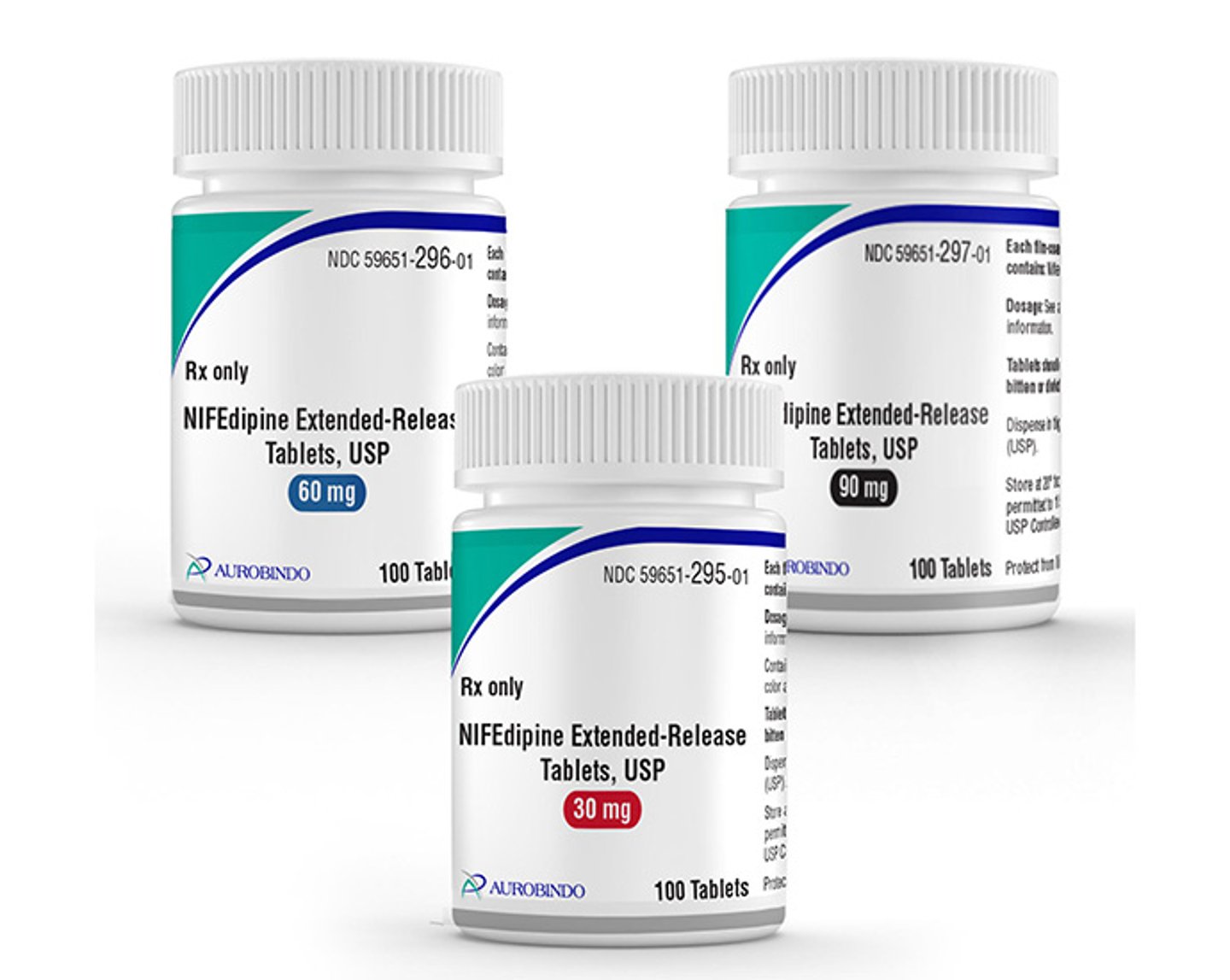
The Food and Drug Administration has approved Aurobindo Pharma’s nifedipine extended-release tablets in three dosage strengths.
The product, a generic of Alvogen’s Adalat CC, is indicated as a treatment for hypertension. Aurobindo’s generic will be available in dosage strengths of 30-, 60- and 90mg.
The market size of the product was $95 million for the 12-month period ended May 2021, according to IQVIA data.