Camber touts 13 generic launches in Q1 2025
Camber is reflecting on the successful release of 13 new generic products in the first quarter of 2025.
“With strong momentum, we are on track to introduce more than 60 new medications this year, strengthening our mission to improve access and affordability across the healthcare landscape,” the company said. “Camber has become a leader in USFDA-approved ANDAs, and we take great pride in offering patients and customers a growing portfolio of complex generic pharmaceutical products. Our capabilities now extend beyond traditional tablets, capsules, and liquids to include specialty products and injectables, enabling us to meet broader industry needs and support even more patient populations.”
In addition to these first-quarter launches, Camber anticipates receiving 54 new ANDA approvals and filing 35 more applications in 2025.
[Related: Camber announces plans for 2025 market intros]
“It’s not just about expanding our portfolio—it’s about strategically delivering the right mix of products that support our customers where they need it most,” said Kon Ostaficiuk, president of Camber Pharmaceuticals. “With the continued innovation of our parent company, Hetero, we’re proud to play a critical role in advancing access to essential medicines across the U.S.”
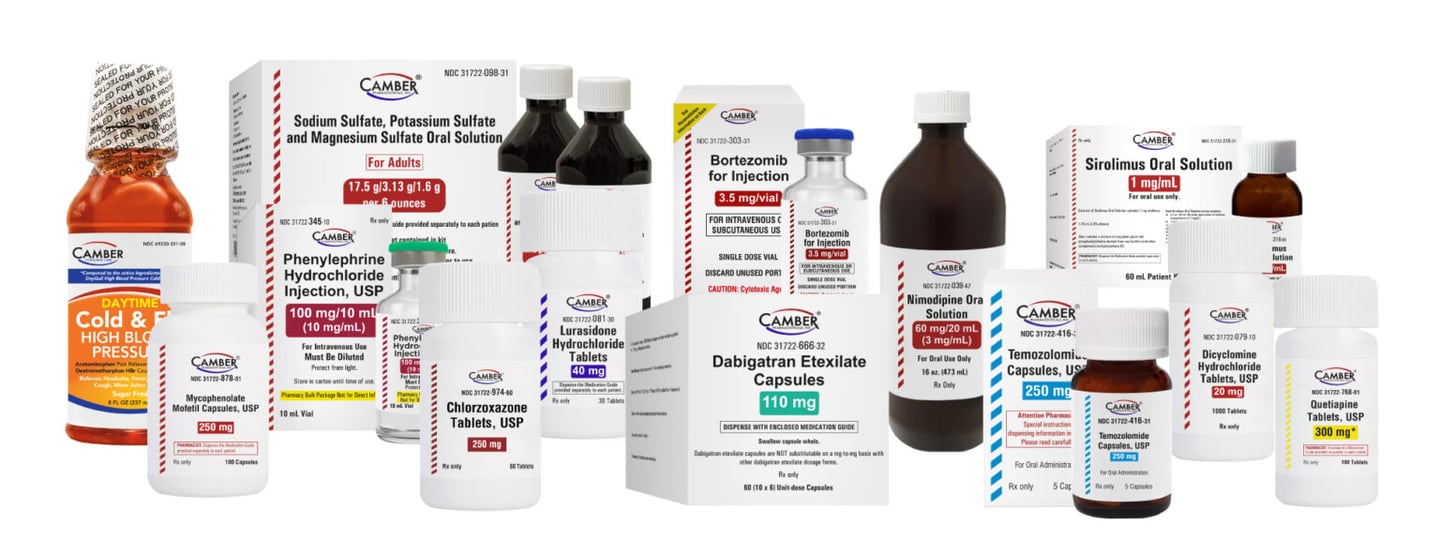
Products introduced in Q1 2025:
- Phenylephrine for Injection (generic for Vazculep)
- Mycophenolate Mofetil Capsules (generic for CellCept)
- Dabigatran Capsule Blister Packs (generic for Pradaxa)
- Chlorzoxazone Tablets (generic for Paraflex)
- Temozolomide Capsules (generic for Temodar)
- Sodium Sulfate-Potassium Sulfate-Magnesium Sulfate Oral Solution (generic for Suprep Bowel Prep Kit)
- Lurasidone HCl Tablets (generic for Latuda)
- Sirolimus Oral Solution (generic for Rapamune)
- Dicyclomine HCl Tablet (generic for Bentyl)
- Bortezomib Injection (generic for Velcade)
- Nimodipine Oral Solution
- Quetiapine Tablets (generic for Seroquel)
- Daytime Cold & Flu HBP (generic for DayQuil HBP Cold & Flu)