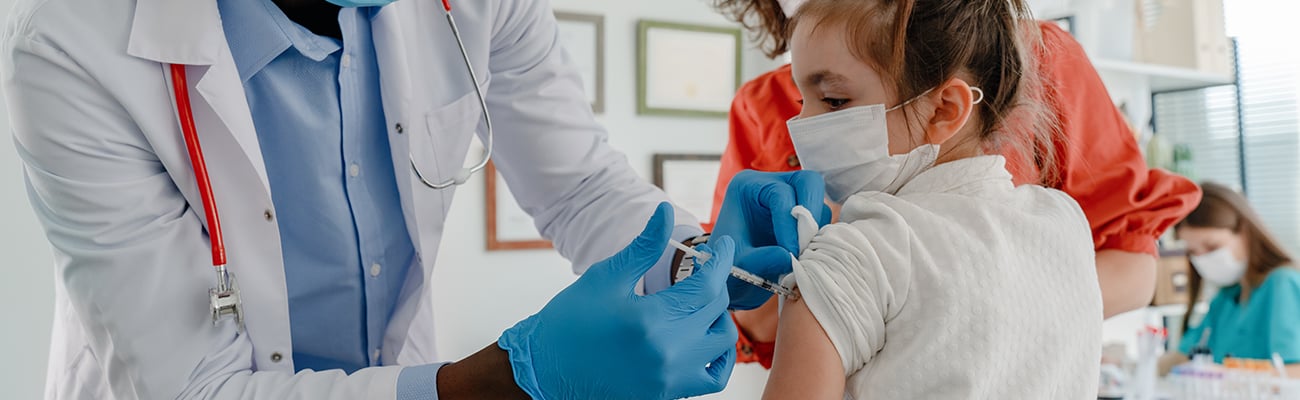
FDA recommends Pfizer’s COVID-19 vaccine for children ages 5 to 11 years old
Vaccine advisers to the Food and Drug Administration voted to recommend a lower dose of Pfizer BioNTech’s COVID-19 vaccine in children age 5 to 11 years old, paving the way for the shots to be offered to 28 million young children in the United States, according to a CNN report.
Pfizer has cut its vaccine to one-third of the adult dose for children aged 12 years old and under.
Members of the FDA’s Vaccines and Related Biological Products Advisory Committee agreed that the benefits of vaccinating younger children appeared to outweigh the risks, but some members appeared troubled about voting to vaccinate a large population of younger children based on studies of a few thousand, the report said.
"It is reassuring to me that we are giving a lower dose," said Dr. Paul Offit, a vaccine expert at Children's Hospital of Philadelphia.
[Read more: White House details COVID-19 vaccine plan for children ages 5 to 11]
"I am just worried that if we say yes, then the states are going to mandate administration of this vaccine for children to go to school and I do not agree with that," said Dr. Cody Meissner, a professor of pediatrics at Tufts University School of Medicine. "I think that would be an error at this time."
But Dr. Amanda Cohn of the Centers for Disease Control and Prevention reminded the committee that children have died of COVID-19. According to CDC, more than 700 children aged 18 years old and under have died of COVID-19. "We don't want children dying of COVID," she said. "And we don't want children in the ICU."
The FDA had said that, under most of the scenarios it projected, the benefits of vaccinating younger children would outweigh any risks and Pfizer said clinical trials showed the vaccine was more than 90% effective in preventing symptomatic infection in children.
[Read more: Pharmacy chains offering COVID-19 vaccine to adolescents]
The FDA will now take the committee’s vote under consideration. Then vaccine advisers to the CDC will meet from Nov. 2 to 3, to discuss the decision and decide whether to recommend that kids get the vaccine. The final word will lie with CDC director Dr. Rochelle Walensky, and vaccination could begin next week of she gives the go-ahead.
The federal government has a plan in place for delivering the smaller-sized vaccines to pediatricians’ offices, pharmacies and other venues across the country.