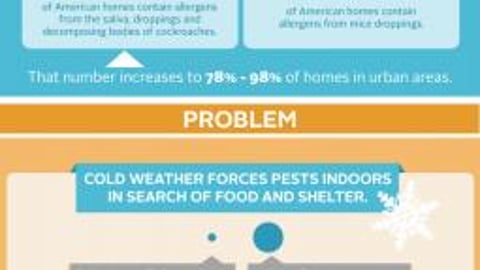
-
Study: Vitamin D supplementation may augment chronic hive therapy
OMAHA, Neb. — A study by researchers at the University of Nebraska Medical Center found that vitamin D as an add-on therapy could provide some relief for chronic hives, a condition with no cure and few treatment options.
Jill Poole, associate professor in the UNMC Department of Internal Medicine, was principal investigator of a study in the Feb. 7 edition of the Annals of Allergy, Asthma and Immunology. The two-year study looked at the role of over-the-counter vitamin D3 as a supplemental treatment for chronic hives.
-
Reckitt Benckiser talks about Mucinex, Airborne line extensions
SLOUGH, England — Reckitt Benckiser has line extended its Mucinex franchise into the year-round allergy sector with Mucinex Allergy, Heather Allen, Reckitt Benckiser EVP category development, told analysts Wednesday.
"Super fantastic launch in the U.S.," she said. "Allergy is a $2.5 billion market in the U.S. More than 75% of Mucinex users also take an allergy treatment, and now, we're able to offer them Mucinex Allergy, maximum strength, non-drowsy antihistamine, acts fast and lasts for 24 hours."

 The January/February 2014 Vitamins and Supplements Ingredient Guide breaks down the indication, ingredients, purpose and dosage of Vitamints Immune, Centrum Specialist Immune Support, Alive! Children's Multi-Vitamin Gummy, Natrol's Melatonin, VitaMelts Multi, TruBiotics, Align and Culturelle Digestive Health Capsules.
The January/February 2014 Vitamins and Supplements Ingredient Guide breaks down the indication, ingredients, purpose and dosage of Vitamints Immune, Centrum Specialist Immune Support, Alive! Children's Multi-Vitamin Gummy, Natrol's Melatonin, VitaMelts Multi, TruBiotics, Align and Culturelle Digestive Health Capsules. The November/December 2013 Cough, Cold and Flu Ingredient Guide breaks down the indication, ingredients, purpose and dosage of Robitussin Maximum Strength, NyQuil Severe Cold & Flu, Theraflu Warming Caplets, Coricidin HBP Cold & Flu, Tylenol Cold & Flu Severe, Hyland's Defend Severe Cold & Flu, Similasan Mucus Relief and Splintek Night Guard.
The November/December 2013 Cough, Cold and Flu Ingredient Guide breaks down the indication, ingredients, purpose and dosage of Robitussin Maximum Strength, NyQuil Severe Cold & Flu, Theraflu Warming Caplets, Coricidin HBP Cold & Flu, Tylenol Cold & Flu Severe, Hyland's Defend Severe Cold & Flu, Similasan Mucus Relief and Splintek Night Guard.