-
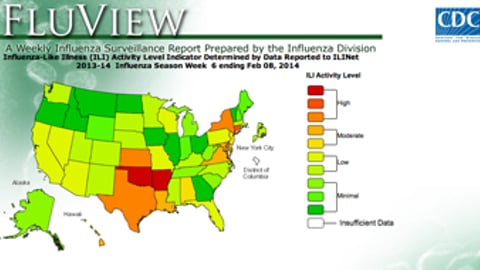
CDC: Working-age Americans hit hard by flu, only 1-in-3 getting flu shot
ATLANTA — Only one-third of adults between the ages of 18 and 64 have gotten their flu shot this season, which is a contributing factor to why this year's flu activity has hit young adults particularly hard, according to Tom Frieden, director of the Centers for Disease Control and Prevention.
-
WSJ: Pills of the future to replace injection as specialty drug delivery mechanism
NEW YORK — The Food and Drug Administration recently approved two "robotic pills," or pills that place an image camera or ingestible sensors into the gastrointestinal tract, according to a report published earlier this week by the Wall Street Journal.
Other robotic pills still in development include one backed by Google — a pill that would replace injectable drugs, the report noted.