-
Gilead voluntarily recalls one lot of injected AIDS-related infection drug
FOSTER CITY, Calif. — Drug maker Gilead Sciences has recalled a single lot of an injected drug used to treat an opportunistic infection in AIDS patients due to the presence of foreign matter in some vials, the company said.
Gilead announced the voluntary recall of lot B120217A of Vistide (cidofovir), an injected drug used to treat cytomegalovirus retinitis in patients with AIDS, due to particulate matter.
-
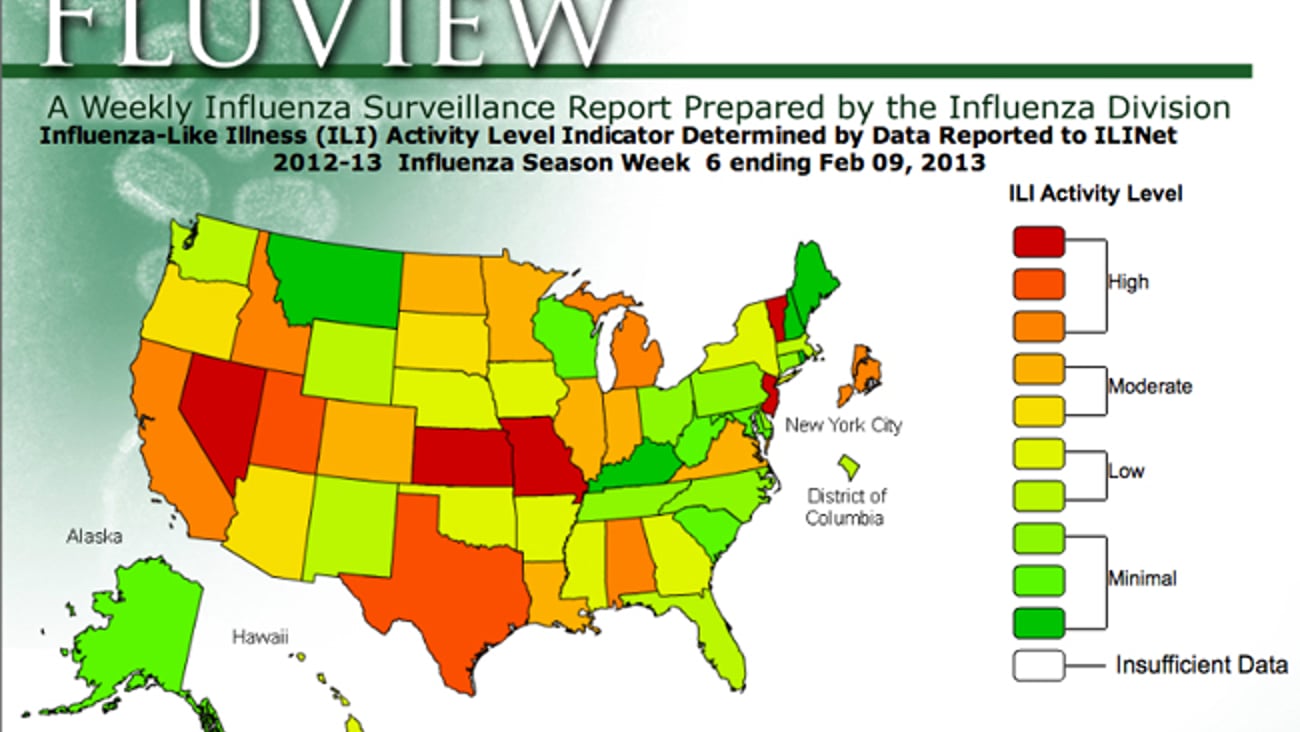
CDC: Flu activity drops to 3.2%, still above national baseline of 2.2%
ATLANTA — For the week ended Feb. 9, influenza activity decreased to 3.2% — still above the baseline of 2.2%, but an indicator this year's flu season is on its way out.
Eleven states and New York City were still experiencing high influenza-like illness activity, while 10 states reported moderate activity.