-
Teva gets FDA clearance for QVAR RediHaler
SILVER SPRING, Md. — The Food and Drug Administration has approved Teva’s QVAR RediHaler (beclomethasone dipropionate HFA), the company announced Monday. The QVAR RediHaler is a breath-actuated inhaler for the maintenance of asthma as a prophylactic treatment in patients ages 4 years and older.
-
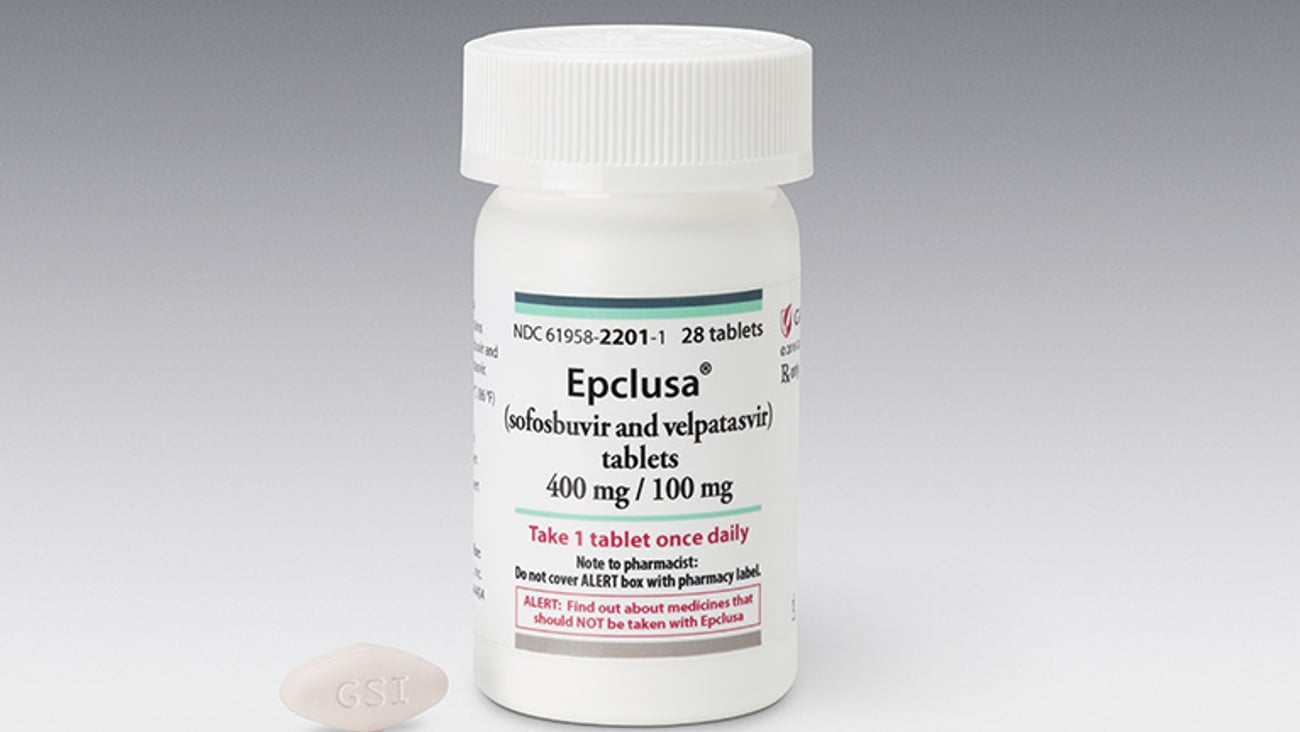
Gilead’s Epclusa gets expanded indication
SILVER SPRING, Md. — Gilead Sciences’s Epclusa single-tablet hepatitis C treatment this week got approval from the Food and Drug Administration for an expanded indication. The drug is now approved for use in patients with hepatitis C that are co-infected with HIV.