-
Enabling patient-facing care: Pharmacists at the top of their licenses
Pharmacists still are waiting for the handcuffs to come off. That’s the consensus of industry leaders who are frustrated with the challenges of getting reimbursements for a wider range of services these professionals can perform. It’s a topic at the center of enabling patient care in community-based pharmacy.
“We talk about pharmacists being able to practice at the top of their license,” said CVS Health executive vice president of retail pharmacy and supply chain Kevin Hourican.
-
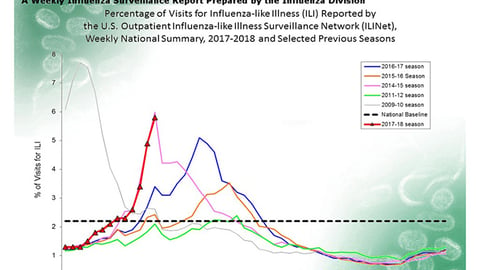
Majority of seniors want LTC and nursing home staff inoculated against flu, poll finds
As flu season swings into high gear, a new poll suggests that nursing homes and other long-term care facilities should be doing more to get their staff and patients vaccinated before it's too late. Nearly three-quarters of people over age 50 surveyed in a new poll say that all staff in such facilities should definitely be required to get the flu vaccine. More than 60% also say that all patients in nursing homes and assisted living should definitely get vaccinated too.