-
Rising Pharmaceuticals launches generic Sustiva
Aceto’s generics subsidiary Rising Pharmaceuticals has introduced a generic of Bristol Myers Squibb’s Sustiva (efavirenz) capsules. The drug is indicated to treat HIV-1 infections in adults and pediatric patients.
The Port Washington, N.Y.-based company’s generic will be available in 50- and 200-mg dosage strengths. The product had U.S. sales of roughly $4.21 million for the 12 months ended October 2017, according to IQVIA data.
-
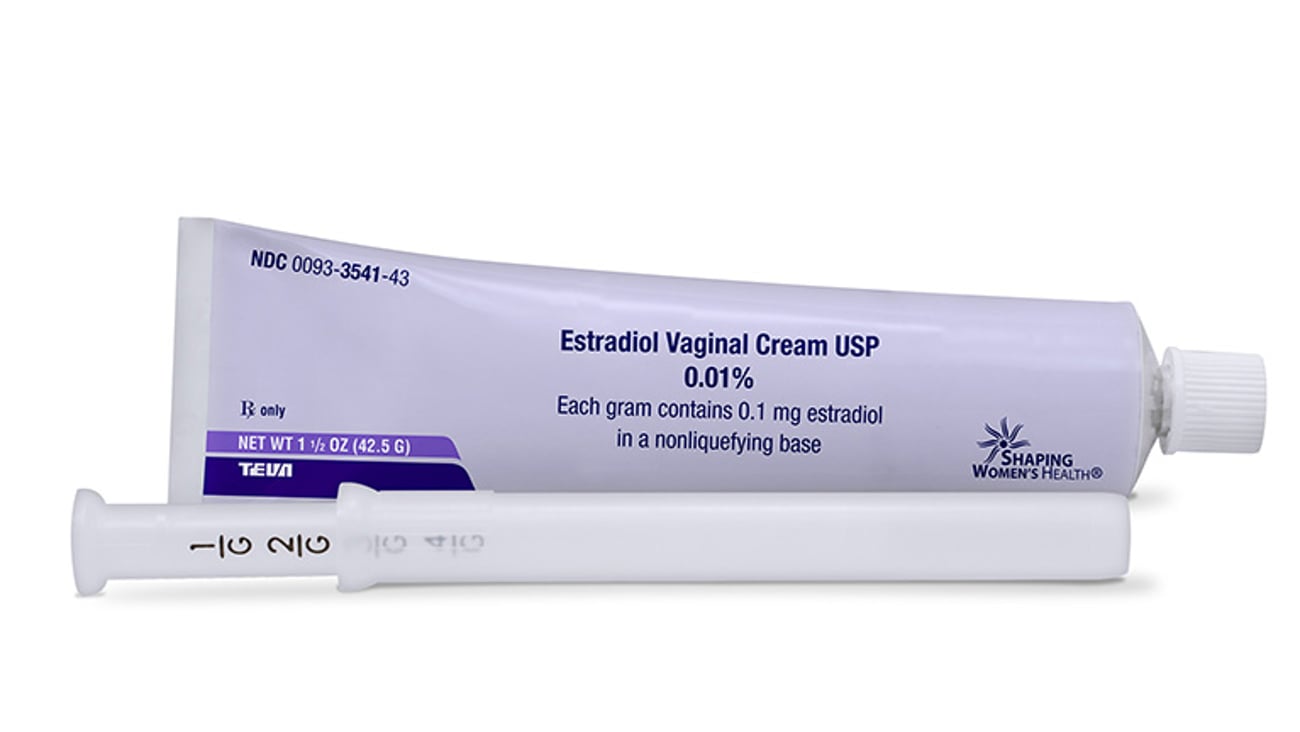
Teva intros authorized Estrace Cream generic
Teva has launched its authorized generic of Allergan’s Estrace Cream (estradiol vaginal cream, 0.01%). The addition to Teva’s women’s health portfolio is indicated to treat moderate-to-severe symptoms of vulvar and vaginal atrophy resulting from menopause.
The drug had U.S. sales of roughly $426 million for the 12 months ended August 2017, according to IQVIA data.