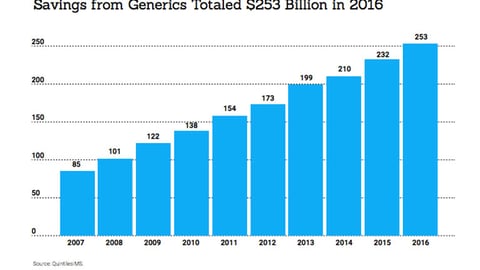
-
FDA approves Aurobindo’s Renvela generic
EAST WINDSOR, N.J. — Aurobindo Pharma USA on Monday announced that its had received Food and Drug Administration approval for its generic of Genzyme’s Renvela (sevelamer carbonate for oral suspension).
The drug is indicated for the control of serum phosphorus in patients with chronic kidney disease.
Aurobindo’s generic will be available in 08.- and 2.4-g pouches. The drug had a market size of $139.9 million for the 12 months ended April 2017, according to QuintilesIMS data.
-
Amneal’s Zetia generic gets launch
BRIDGEWATER, N.J. — Amneal Pharmaceuticals on Thursday announced the introduction of its generic of Zetia (ezetimibe) tablets.
Amneal’s generic will be available in 10-mg dosage strength. The company is distributing the generic in 30-, 90- and 500-count bottle sizes, and has begun shipping the product from its Kentucky distribution center.
Zetia had annual U.S. sales of approximately $2.7 billion for the 12 months ended April 2017, according to QunitilesIMS data.