-
Flush generics industry eyes new openings
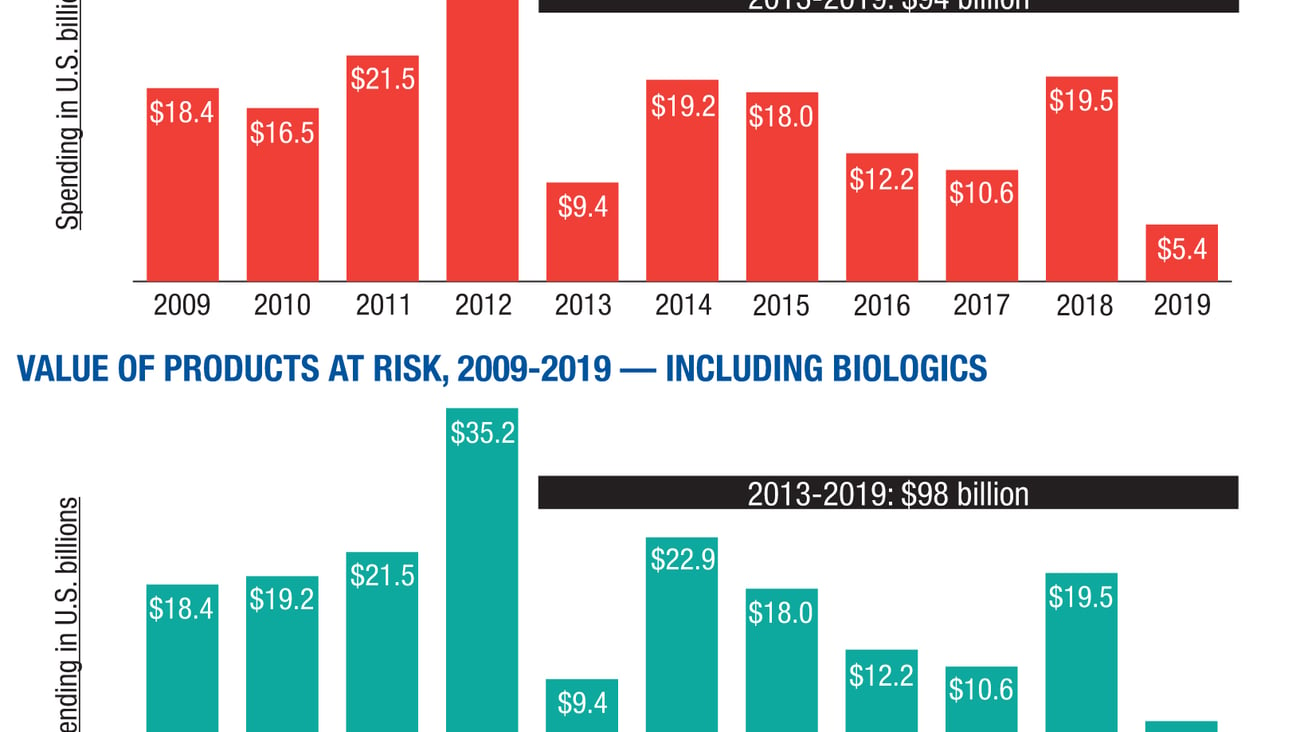
The stampede of big-selling branded medicines that hurtled off the patent cliff in 2011 and 2012 has slowed to a relative trickle. But with generic drugs now accounting for 86% of all drugs dispensed in the United States, according to IMS Health, the me-too drug market remains a powerful growth industry and one of the few segments within the pharmaceutical arena, along with specialty drugs, that continues to drive overall market momentum for prescription medicines.
-
Actavis extends agreement to market, distribute generic Concerta
DUBLIN — Actavis has reached an agreement with Janssen Pharmaceuticals to continue supplying the authorized generic version of Janssen's Concerta (methylphenidate hydrochloride extended-release tablets), the company announced.
Under the terms of the agreement, Janssen Pharmaceuticals will continue to manufacture and supply Actavis with all dosage strengths of the generic drug, and Actavis will continue to market and distribute the drug in the United States. This agreement runs until Dec. 31, 2017.