-
FDA commissioner blogs regarding challenges facing Indian drug manufacturers
SILVER SPRING, Md. — In a blog post updated Friday, Food and Drug Administration commissioner Margaret Hamburg addressed two challenges facing Indian drug manufacturers — approval times for abbreviated new drug applications and quality inspections.
-
Mapping out the next generics wave
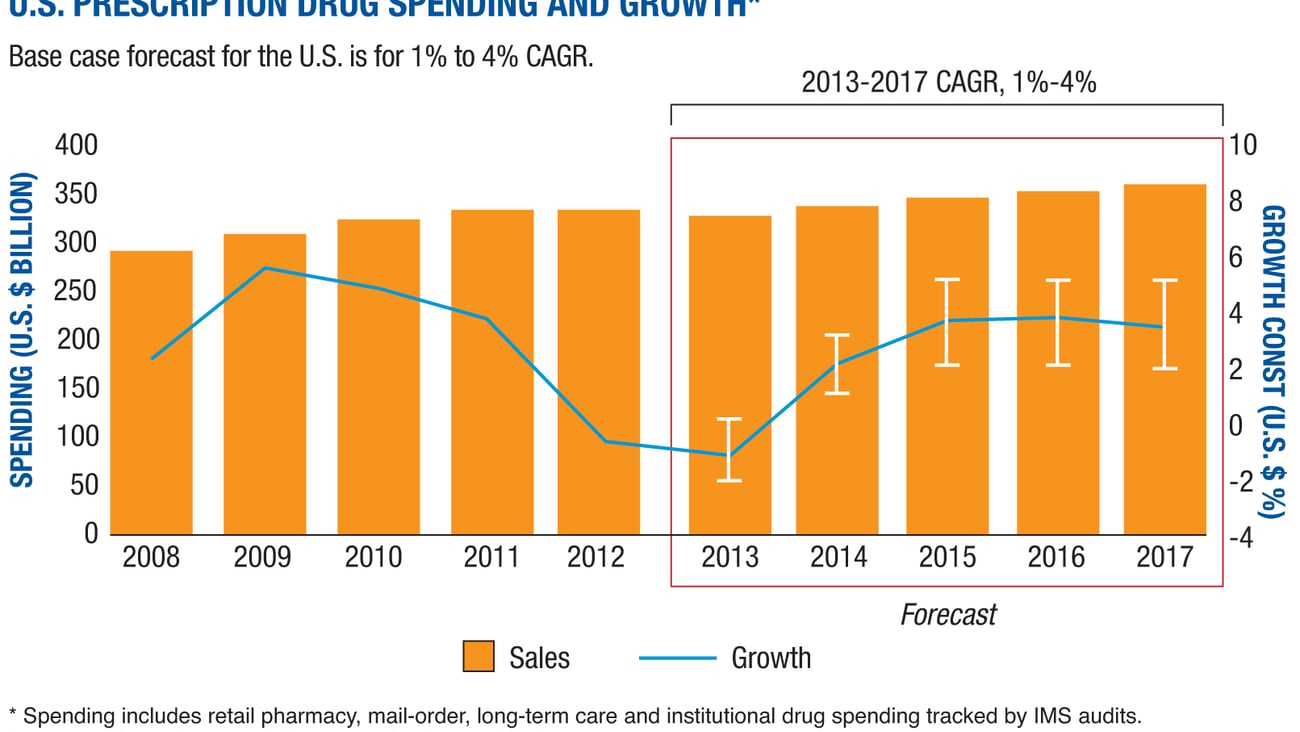
From 2012 to 2017, global spending on medicines will increase from $205 billion to $235 billion, according to IMS Health. By 2017, 36% of the spend will be on generics, a number that is 9% more than the percentage in 2013.
As a result of the patent cliff, generic drug manufacturers have thrived while branded pharmaceutical manufacturers have suffered. Branded pharmaceutical manufacturers are expected to suffer even more in the coming years, as many more important patents will lose exclusivity.