-
GDUFA tackles backlog of drug approval applications, FDA hires more staff
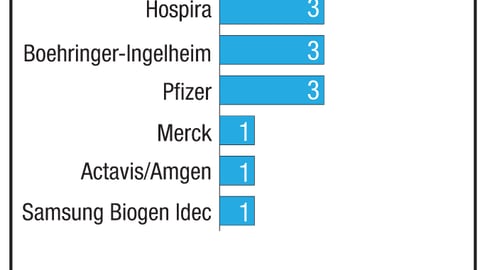
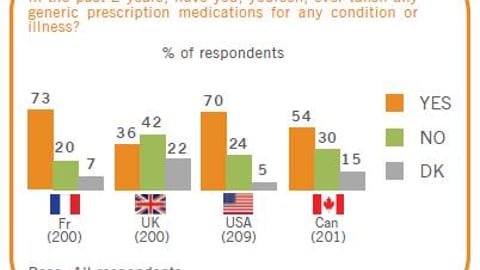
For the Food and Drug Administration’s Office of Generic Drugs, one of the biggest stumbling blocks has been its significant backlog of generic drug approval applications. But it’s steadily making progress in addressing the problem.
When Congress approved a reauthorization of the Prescription Drug User Fee Act that included the Generic Drug User Fee Amendments last year, that backlog included about 2,500 applications. But thanks to GDUFA, the agency has managed to clear muchof it.