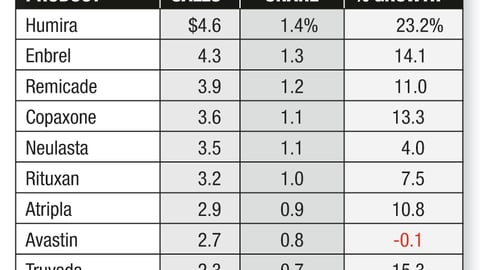
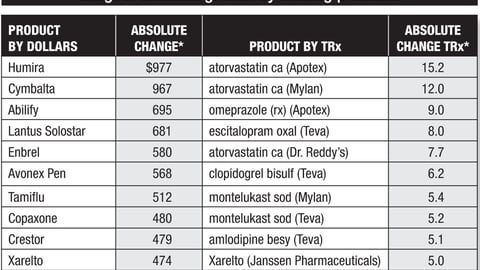
-
FDA exceeds FY2013 GDUFA hiring goals
SILVER SPRINGS, Md. — The Food and Drug Administration has exceeded its goals for hiring new staff as part of a user-fee law for generic drugs, according to an internal memo sent to staff members.
In a memo to staff of the agency's Center for Drug Evaluation and Research, center director Janet Woodcock wrote that for fiscal year 2013, the FDA hired 234 people as part of the Generic Drug User Fee Amendments Human Capital Team. The goal was to hire 231 as part of a three-year push in which it hopes to hire 921, including 461 during fiscal year 2014.