-
Medicare dashboard advances ACA goals for chronic conditions
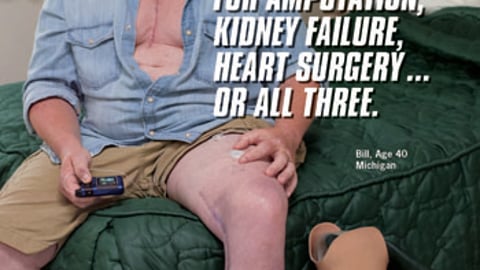
WASHINGTON — A new Medicare Chronic Conditions Dashboard announced today by Marilyn Tavenner, acting administrator of the Centers for Medicare and Medicaid Services (CMS), furthers the Patient Protection and Affordable Care Act’s goals for health promotion and the prevention and management of multiple chronic conditions.