-
PBMS leverage generics as effective cost-saving tool
Pharmacy benefit managers are becoming increasingly adept at leveraging the power of generics to save client healthcare dollars and improve their own standing, reports indicated.
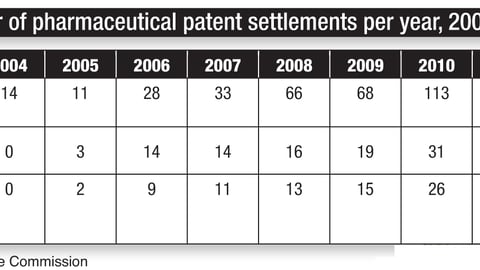
"The opportunity for lowering costs by promoting generics over brands has never been greater, given the unprecedented number of drugs set to lose patent protection over the next few years," noted the "2012 Towers Watson/National Business Group on Health Employer Survey on Purchasing Value in Health Care."