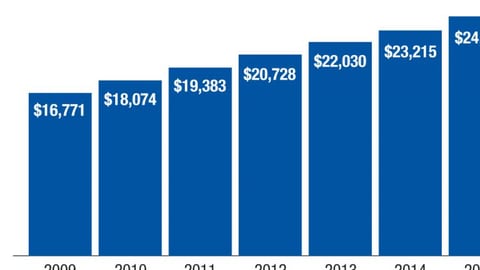
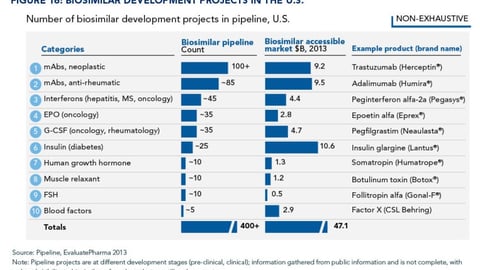
-
CRN begins testing its Supplement Online Wellness Library
WASHINGTON — As part of an initiative to provide a deeper look across the VMS products the dietary supplement industry are bringing to market, nine companies are voluntarily beta-testing the Council for Responsible Nutrition's dietary supplement product registry, which will be branded as the Supplement Online Wellness Library (Supplement OWL).
-
NACDS promotes long-standing retail pharmacy advocate to SVP government affairs and public policy
ARLINGTON, Va. – The National Association of Chain Drug Stores on Thursday promoted Thomas O’Donnell to SVP government affairs and public policy, effective Sept. 7, 2016. O’Donnell has served as VP federal government affairs, since joining the NACDS staff team on July 9, 2012.
In his new capacity, O’Donnell will report to NACDS president and CEO Steven Anderson.