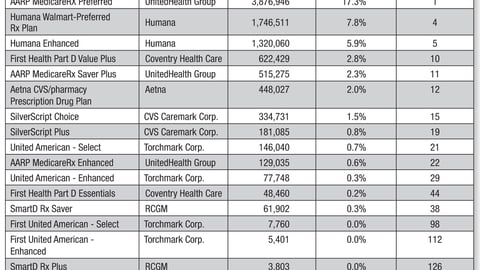
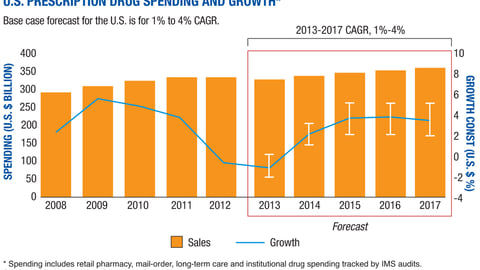
-
Report: Lewis Drug acquires Throndset pharmacies in Minnesota
SIOUX FALLS, S.D. — Regional player Lewis Drug has acquired Throndset pharmacies in Jackson and Lakefield, Minn., according to a local news report.
The transaction is expected to take effect April 7 and, according to the Lakefield Standard, Lewis Drug will retain all of the employees. -
WSJ: FDA to discuss updating monograph system
SILVER SPRING, Md. — The Food and Drug Administration will host a public meeting in March to discuss updating the OTC monograph system, the Wall Street Journal reported Friday.
According to the report, the FDA will consider improving the agency's ability to initiate label changes quickly, allowing for faster innovation from manufacturers and imposing new pediatric dosing limits based on the most recent science.