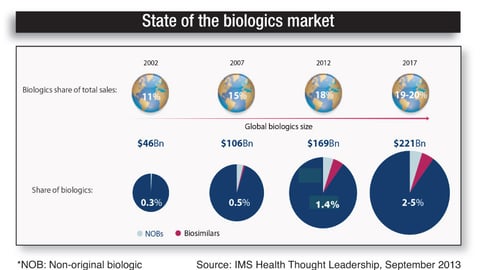
-
Stayhealthy gets license from Health Canada
MONROVIA, Calif. — Regulators in Canada have given a maker of health kiosks the green light to start marketing its products there.
Stayhealthy said Health Canada's Medical Devices Bureau had granted it a license to sell its HealthCenter Kiosk, models 650 and 650-C. The kiosks measure blood pressure, heart rate, total body weight, body-mass index, total body composition and vision and can incorporate data on blood glucose.
-
Gilead's Complera approved for HIV patients already on stable drug regimens
FOSTER CITY, Calif. — The Food and Drug Administration has approved an HIV pill made by Gilead Sciences for patients who are switching from other therapy regimens, the company said.
Gilead said the FDA approved its single-pill regimen, Complera (emtricitabine; rilpivirine; tenofovir disoproxil fumarate) for use in adult patients who have suppressed their infections on stable antiretroviral regimens and are replacing their current regimens. The FDA originally approved the drug in 2011.

 Sales of personal lubricants were down 5.2% to $207.5 million in sales for the 52 weeks ended Oct. 6, according to IRI across total U.S. multi-outlets. A big part of that decrease is due to new Food and Drug Administration regulations that require lubricant manufacturers to submit a 501k medical device application for approval.
Sales of personal lubricants were down 5.2% to $207.5 million in sales for the 52 weeks ended Oct. 6, according to IRI across total U.S. multi-outlets. A big part of that decrease is due to new Food and Drug Administration regulations that require lubricant manufacturers to submit a 501k medical device application for approval.