-
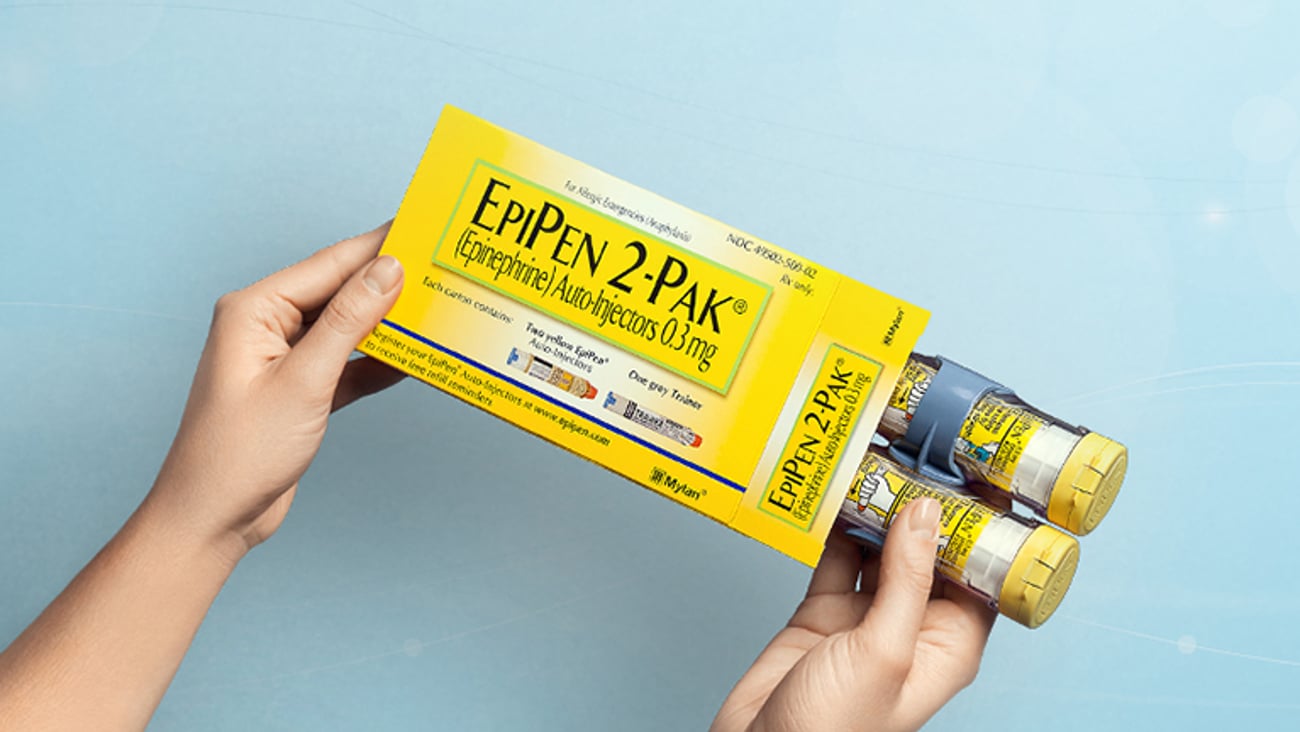
Mylan launches first generic EpiPen Auto-Injector
HERTFORDSHIRE, England - Mylan on Monday announced that its U.S. subsidiary will launch the first generic to EpiPen Auto-Injector (epinephrine injection, USP) at a list price of $300 per generic EpiPen two-pack carton, which represents a discount of more than 50% to the Mylan list price, or wholesale acquisition cost, of the branded medicine.
-
Upsher-Smith announces new distribution, marketing partnership
MAPLE GROVE, Minn. — In an effort to grow its generics pipeline, Upsher-Smith Laboratories on Friday announced a new agreement with an emerging pharmaceutical company.Though Upsher-Smith did not name the company, it said the collaboration was aimed at marketing and distributing a generic central nervous system product that had U.S. sales of $200 million for the 12 months ended June 2016, according to IMS Health data.