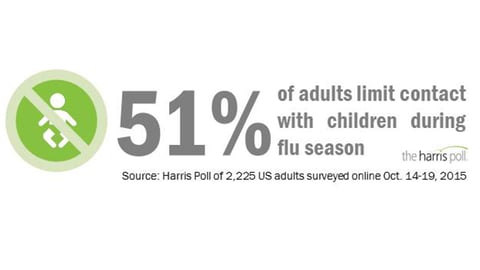
ATLANTA - For the week ended March 19, the Centers for Disease Control and Prevention reported that influenza activity decreased slightly, but remained elevated in the United States. Even so, by all indications the week ended March 12 may have been the peak of the 2015/2016 influenza season.
That means while this season may have started later than is typical, and lasted longer than is typical, the U.s. hasn't experienced a season with as few overall visits for influenza-like illness since the 2011/2012 season.