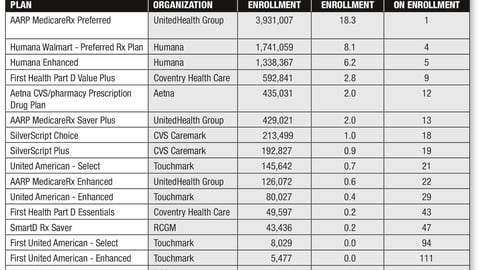
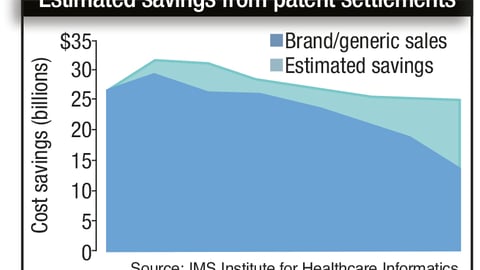
-
Meijer expands mPerks program to include pharmacy rewards for fuel and groceries
GRAND RAPIDS, Mich. — Meijer announced today that it is expanding its mPerks digital coupon program to include pharmacy purchases, allowing customers to personalize their savings and earn rewards in the areas they shop the most whenever they fill a prescription.
The Grand Rapids, Mich.-based retailer launched the mPerks Pharmacy Rewards program this month after reaching 1.5 million mPerks subscribers and saving enrolled customers $21.5 million so far this year.
For every five eligible prescriptions filled, customers can earn one of the following:
-
Teva, Perrigo announce launch of generic temozolomide
 JERUSALEM and ALLEGAN, Mich. — Teva Pharmaceutical Industries Ltd., and Perrigo Co. today announced the launch of the generic equivalent to Temodar (temozolomide). Teva will manufacture, market and distribute the product in the United States, and both companies will equally share in the cost and profitability of the product in the country. Teva was first to file, making the product eligible for 180 days of marketing exclusivity.
JERUSALEM and ALLEGAN, Mich. — Teva Pharmaceutical Industries Ltd., and Perrigo Co. today announced the launch of the generic equivalent to Temodar (temozolomide). Teva will manufacture, market and distribute the product in the United States, and both companies will equally share in the cost and profitability of the product in the country. Teva was first to file, making the product eligible for 180 days of marketing exclusivity.