-
Diplomat's mission: Bridging gap between specialty and traditional Rx
Can one of the nation's premier specialty pharmacies maintain its focus on each patient it serves while simultaneously smashing its own growth records and reaching national power-player status in the rarified world of specialized medication services?
-
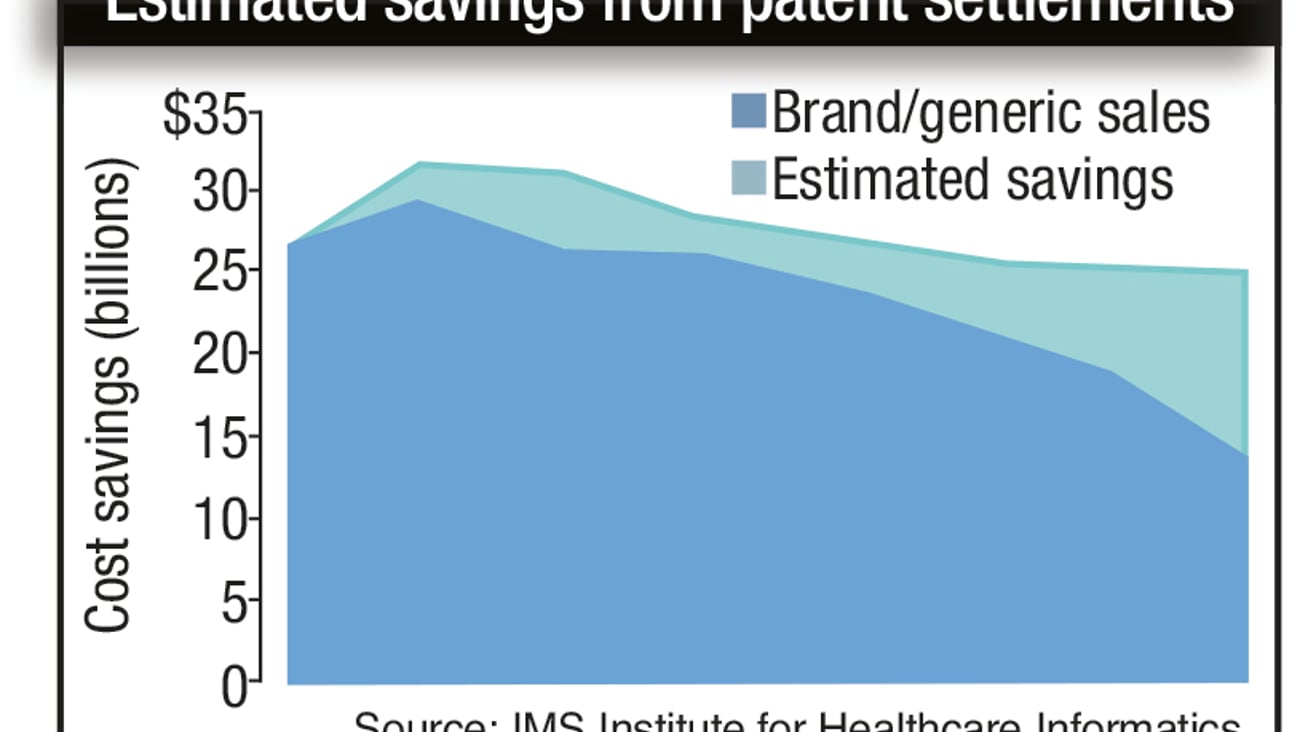
IMS study: Settlements save healthcare system, federal government billions
The Supreme Court usually has a lot on its plate in any given year, but this year's term included a pretty big case for the pharmaceutical industry: the Federal Trade Commission v. Actavis, which concerned legal settlements between branded and generic drug makers that often occur when the latter attempts to market a generic drug before the former's patents have expired.