-
FDA approves BDSI's NDA for Bunavail
RALEIGH, N.C. — BioDelivery Sciences International on Monday announced that the Food and Drug Administration approved its new drug application for Bunavail (buprenorphine and naloxone) buccal film (CIII). The drug is used as a maintenance treatment of opioid dependence. The company noted that it also should be used as part of a complete treatment plan to include counseling and psychosocial support.
Bunavail is the first and only formulation of buprenorphine and naloxone for buccal (inside of the cheek) administration, BDSI said.
-
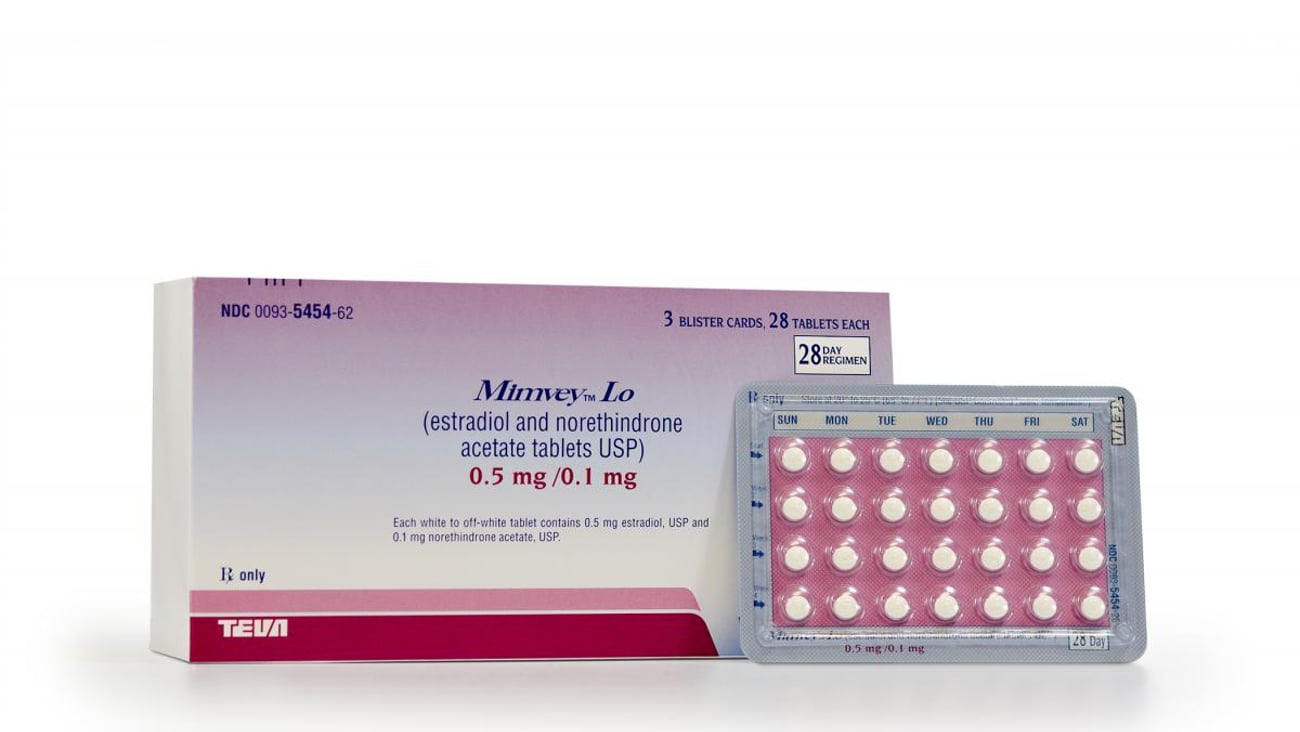
Teva announces generic Activella tabs
NORTH WALES, Penn. — Teva Pharmaceuticals announced the launch of Mimvey Lo (estradiol and norethindrone acetate tablets, USP), the generic equivalent to Activella tablets. The drug is used to treat certain symptoms of menopause.
"Our customers count on Teva for a continuous supply of new generic products,” said Maureen Cavanaugh, SVP U.S. Generics Sales & Marketing. “With the launch of Mimvey Lo, we add another quality product to our broad line of affordable generic pharmaceuticals.”