-
Quest Products to relaunch Alocane Emergency Burn Gel
GURNEE, Ill. — Quest Products on Friday announced the addition of a gel formulated for emergency burn relief, Alocane Emergency Burn Gel, to its current line of products. Previously a top-selling product on retail shelves in the 80's and 90's, it was taken off the market due to financial difficulties facing the parent company, First Pharmaceutical.
-
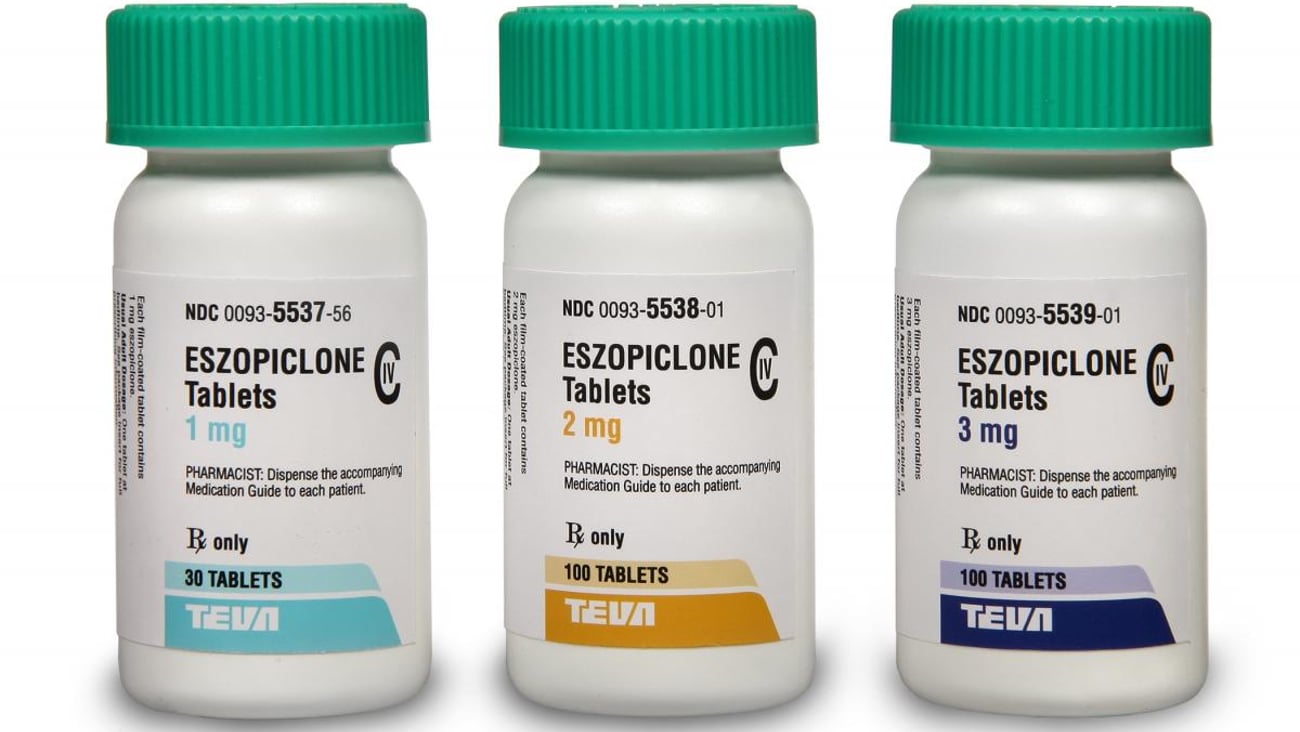
Teva launches generic Lunesta in the U.S.
JERUSALEM — Teva Pharmaceutical Industries announced the launch of a generic equivalent to Lunesta (eszopiclone tablets) in 1-,2- and 3-mg form in the United States. The drug is used to treat insomnia.
Lunesta tablets, which are marketed by Sunovion Pharmaceuticals, had annual sales of $852 million in the United States as of December 2013, according to IMS data.