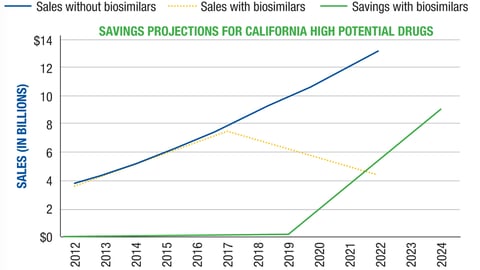
-
AANP urges legislators to follow FTC lead against practice restrictions
AUSTIN, Texas — The American Association of Nurse Practitioners, a national professional membership organization for nurse practitioners of all specialties, is calling on state lawmakers to consider the consequences of undue restrictions on APRNs, or nurse practitioners, as recommended by the Federal Trade Commission.