-
Sandoz starts late-stage trial of Humira biosimilar
HOLZKIRCHEN, Germany — Drug maker Sandoz has started a late-stage clinical trial of a biotech drug used to treat autoimmune disorders, the company said.
Sandoz, the generics division of Swiss drug maker Novartis, announced the start of a phase-3 clinical trial of biosimilar adalimumab. The drug is a version of AbbVie's Humira, used to treat rheumatoid arthritis, psoriasis and Crohn's disease.
-
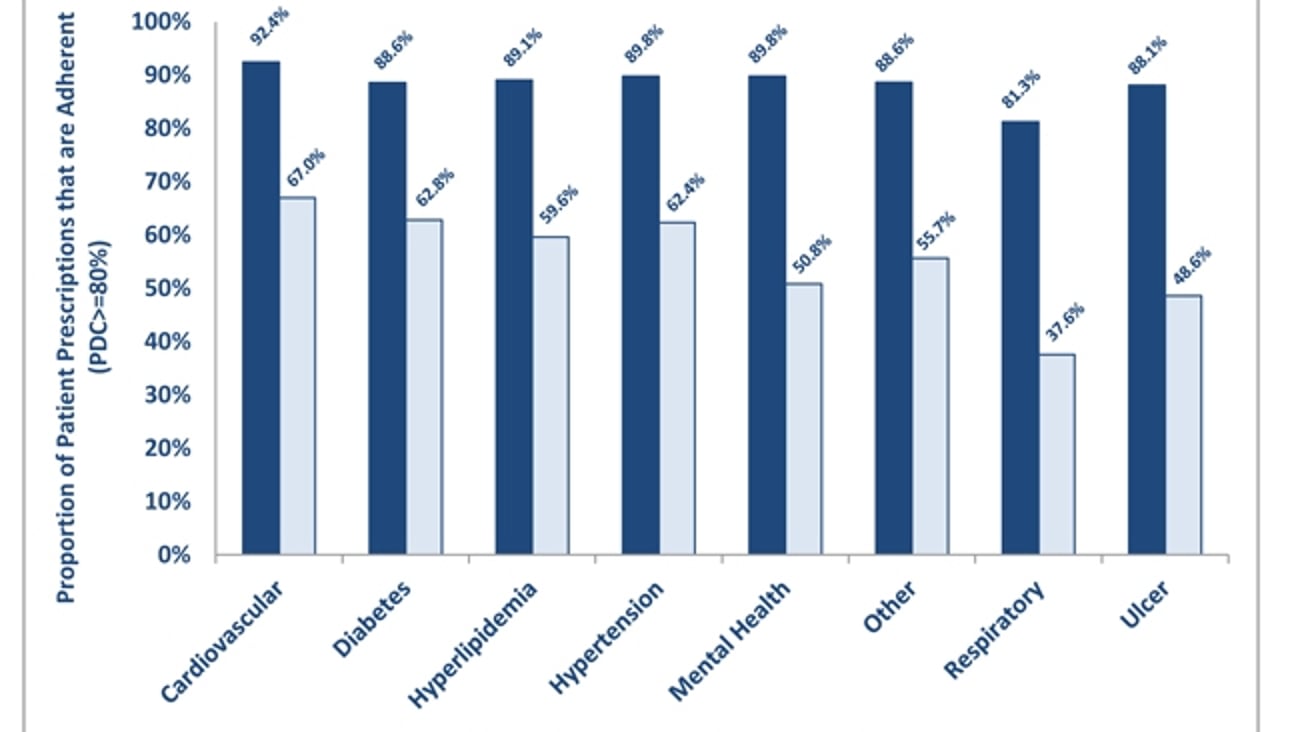
Study from NCPA sheds new light on med synchronization programs
ALEXANDRIA, Va. — Patients who opt-in to medication synchronization programs offered through their community pharmacy average more than 100 additional days on therapy per year and are 30% more likely to take their medication as prescribed than patients not enrolled in a synchronization program, according to the results of a new study project released Wednesday that was conducted by the National Community Pharmacists Association.