-
Alternate sites woo payers
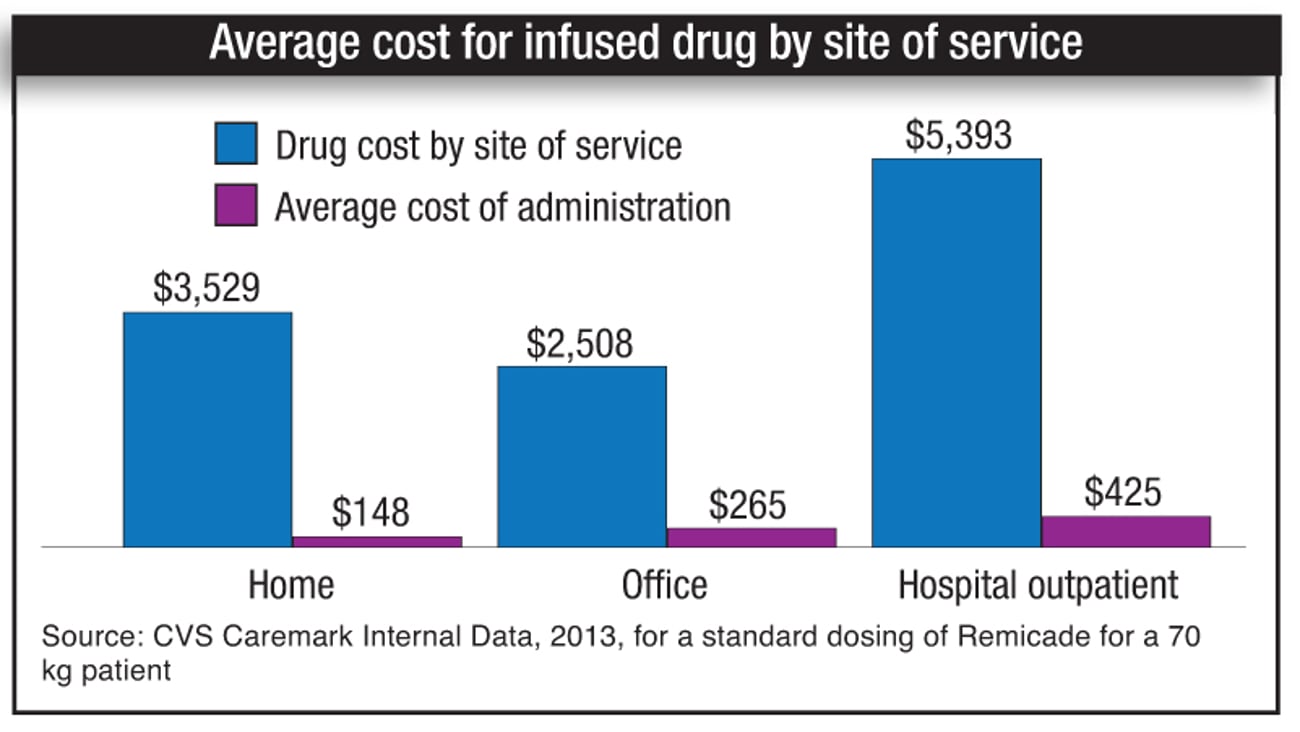
Administering high-touch, expensive and complex medications to patients intravenously in their homes — or in a setting other than a hospital — is essentially a large-scale bid to “reduce costs by transferring non-self-administered drugs to the most cost-effective and clinically appropriate site of care,” said pharmacist Michael Einodshofer, senior director of specialty strategy and innovation at Walgreens Specialty Pharmacy.