-
i-Health products offer women more relief options
CROMWELL, Conn. — i-Health’s Estroven has been dialing up sales tracked within feminine pain relievers. The core brand generated $16.2 million within the last 52 weeks ended Aug. 11 across U.S. multi-outlets, according to IRI. The company’s launch of Estroven Energy has generated another $3.9 million. July brought more innovation to the category with the launch of Estroven Weight Management, a supplement specifically formulated to deliver on a core benefit of helping peri/menopausal women manage their weight.
-
Health Mart’s operations chief tracks build-up in local support
Like politics, retailing is local. That inescapable fact is behind the ongoing build-up of Health Mart’s local marketing and patient care initiatives as McKesson shifts more store support muscle to the more than 3,100 independent pharmacies allied with the Health Mart network through its Local Marketing Support Program.



 Sales of body care products have been on the downslide in recent years, according to research, likely because of consumers turning away from nonessential purchases amid a weak economy. However, times appear to be changing.
Sales of body care products have been on the downslide in recent years, according to research, likely because of consumers turning away from nonessential purchases amid a weak economy. However, times appear to be changing.



 Higher-end products are where the beer category is headed. “We’re seeing a lot of high-end segments continuing to steer the ship,” said Dan Wandel, SVP at IRI. “We’re seeing higher-priced products driving the category.”
Higher-end products are where the beer category is headed. “We’re seeing a lot of high-end segments continuing to steer the ship,” said Dan Wandel, SVP at IRI. “We’re seeing higher-priced products driving the category.”
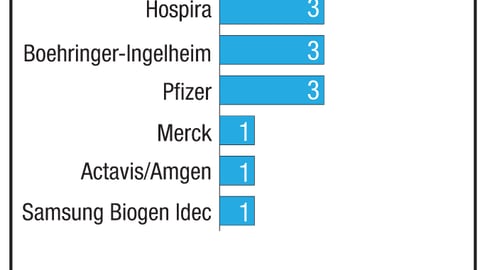

 Many of the larger probiotic brands are experiencing significant growth. i-Health’s Culturelle generated $61.6 million in sales on a growth rate of 44.5% for the 52 weeks ended Aug. 11, according to IRI data across U.S. multi-outlets. And sales of Bayer’s Phillips Colon Health were up 18.5% to $58.7 million.
Many of the larger probiotic brands are experiencing significant growth. i-Health’s Culturelle generated $61.6 million in sales on a growth rate of 44.5% for the 52 weeks ended Aug. 11, according to IRI data across U.S. multi-outlets. And sales of Bayer’s Phillips Colon Health were up 18.5% to $58.7 million.