-
FDA approves generic versions of Aciphex
SILVER SPRING, Md. — The Food and Drug Administration has approved six generic versions of a drug used to treat gastroesophageal reflux disease, or GERD, the agency said Friday.
The FDA announced the approval of the first generic versions of Eisai's Aciphex (rabeprazole sodium) delayed-release tablets for patients aged 12 and older. The generic products are made by Dr. Reddy's Labs, Kremers Urban Pharmaceuticals, Lupin Pharmaceuticals, Mylan Pharmaceuticals, Teva Pharmaceuticals USA and Torrent Pharmaceuticals.
-
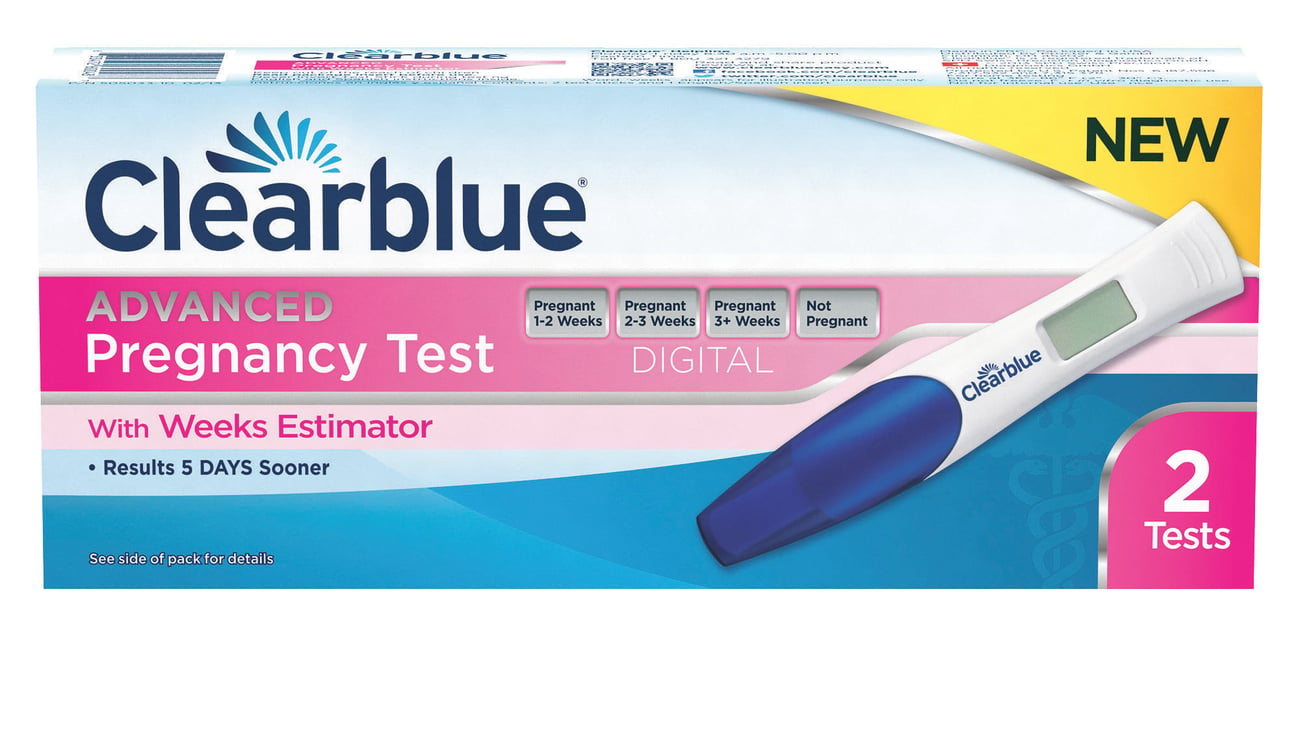
At-home test provides consumers down-to-the-week results
CINCINNATI — Procter & Gamble in August launched its Clearblue Advanced Pregnancy Test with Weeks Estimator — the first and only pregnancy test that also provides an estimate of time since ovulation for women testing pregnant. The introduction will play brand dividends — before the new product’s sales could be tracked, Clearblue pregnancy test kits were already up 23.6% to $39.5 million, across U.S. multi-outlets for the 52 weeks ended Sept. 8, according to IRI.