-
2017 considered one of longest- and latest-running flu seasons
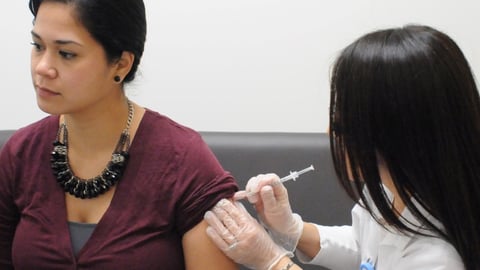
ATLANTA — The 2016/2017 influenza season is still ongoing. According to the latest statistics from the Centers for Disease Control and Prevention, flu incidence is still tracking above the national baseline, making this year both the longest-running season and the latest-running season in recent memory.
-
CVS’ MinuteClinic teams with VA to expand health care access for veterans
PHOENIX — MinuteClinic, the retail medical clinic of CVS Health, is joining forces with the Dept. of Veterans Affairs, the Phoenix VA Health Care System and TriWest Healthcare Alliance for an initiative that will expand access to high quality and convenient health care services for veterans in Phoenix and surrounding communities.